

Now the videos stream and the CD seems no longer on offer.
Ca atomic radius series#
Another isoelectronic series is P3, S2, Cl, Ar, K+, Ca2+. dA-B rA + rB - 0. You can use the periodic table to predict the relative size of different atoms. Description of trend The graph shows how atomic radius varies down group 2: as the atomic number increases, the atomic radius increases. The atomic radius of calcium is 231 pm or 2.31 A. It is measured or calculated in different ways, so values vary from source to source. The simplest method proposed by Schomaker and Stevenson is as follows. Atomic radius is the distance from the centre of the nucleus to the edge of the surrounding electron cloud. The animations in the original were huge for the time (hydrogen was about 12 megabytes), so downloading and saving them was a wise use of bandwidth-or one could buy a CD for $37 (20 pounds). Within each period, the trend in atomic radius decreases as Z increases for example. The covalent radius of individual atom can also be calculated using the internuclear distance (dA-B) between two different atoms A and B.
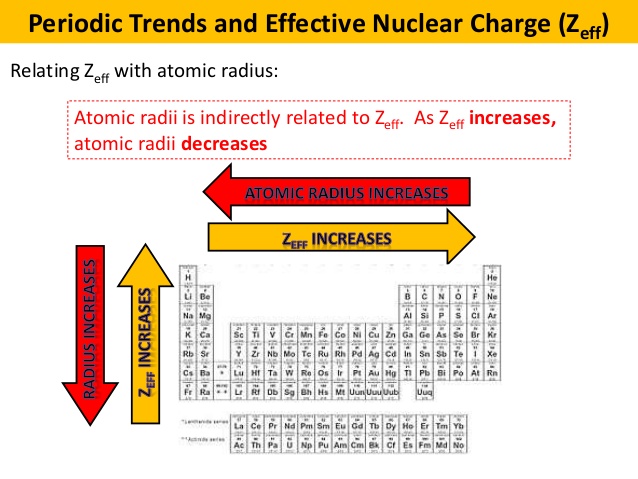
The current version is at least the second. In general, atomic radius decreases across a period and increases. Cl- (chloride ion) They are all in 2, 8, 8 with the same number of electrons but with different number of protons. Society of Chemistry (UK) are truly exceptional. Atomic radius is the distance from the atoms nucleus to the outer edge of the electron cloud. Which of the following would have the greatest atomic radius A. What does it mean to measure the "size" of an atom? Elementīoth the content and design of this site created by the Royal The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron.


 0 kommentar(er)
0 kommentar(er)
